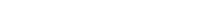I found that the Thompson microphysics scheme (module_mp_thompson.F) used an incorrect constant to calculate the heat of vaporization at a temperature. This error appears in lines 1999, 3206, and 3515 of module_mp_thompson.F (WRF/phys/module_mp_thompson.F at master · wrf-model/WRF).
The code is shown below.
Where lvap(k) is the heat of vaporization per unit mass of water at a temperature of tempc (in degrees Celsius) in layer k, lavp0 is the heat of vaporization per unit mass of water at 0 degrees Celsius. 2106.0 and 4218.0 are the specific heat of water vapor and water at 0 degrees Celsius, respectively. But 2106.0 should be the specific heat of ice at 0 degrees Celsius. At 0 degrees Celsius, the specific heat of water vapor should be about 1858.7 J/kg/K (Water Vapor - Specific Heat vs. Temperature).
The calculation of lvap(k) using Equations 2 and 3 according to the principle of Equation 1 is listed in Table 1.
Table 1 shows that the lvap(k) calculated by Equation 3 are closer to the values given by The Engineering Toolbox (Water - Heat of Vaporization vs. Temperature).
The code is shown below.
lvap(k) = lvap0 + (2106.0 - 4218.0)*tempc (J/kg/K)(1)
Where lvap(k) is the heat of vaporization per unit mass of water at a temperature of tempc (in degrees Celsius) in layer k, lavp0 is the heat of vaporization per unit mass of water at 0 degrees Celsius. 2106.0 and 4218.0 are the specific heat of water vapor and water at 0 degrees Celsius, respectively. But 2106.0 should be the specific heat of ice at 0 degrees Celsius. At 0 degrees Celsius, the specific heat of water vapor should be about 1858.7 J/kg/K (Water Vapor - Specific Heat vs. Temperature).
The calculation of lvap(k) using Equations 2 and 3 according to the principle of Equation 1 is listed in Table 1.
lvap(k) = 2500.9 + (2.106 - 4.218)*(tempc-0.01) (kJ/kg/K) (2)
lvap(k) = 2500.9 + (1.8587 - 4.218)*(tempc-0.01) (kJ/kg/K)(3)
lvap(k) = 2500.9 + (1.8587 - 4.218)*(tempc-0.01) (kJ/kg/K)(3)
Table 1 shows that the lvap(k) calculated by Equation 3 are closer to the values given by The Engineering Toolbox (Water - Heat of Vaporization vs. Temperature).
Table 1. lvap(k) at different temperatures
Temperature °C | Engineering Toolbox kJ/kg/K | lvap(k) by Equation 2 kJ/kg/K | lvap(k) by Equation 3 kJ/kg/K |
0.01 | 2500.9 | 2500.9 | 2500.9 |
2 | 2496.2 | 2496.7 | 2496.2 |
4 | 2491.4 | 2492.5 | 2491.5 |
10 | 2477.2 | 2479.8 | 2477.3 |
14 | 2467.7 | 2471.4 | 2467.9 |
18 | 2458.3 | 2462.9 | 2458.5 |
20 | 2453.5 | 2458.7 | 2453.7 |
25 | 2441.7 | 2448.1 | 2441.9 |
30 | 2429.8 | 2437.6 | 2430.1 |
34 | 2420.3 | 2429.1 | 2420.7 |
40 | 2406.0 | 2416.4 | 2406.6 |
44 | 2396.4 | 2408.0 | 2397.1 |
50 | 2381.9 | 2395.3 | 2383.0 |
|